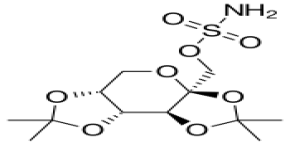
Topiramate
-
API Product :
Topiramate
-
CEP :
-
-
WCC :
?
-
Therapeutic Use :
All Other Therapeutic Products; Analgesics; Lipid-regulating/anti-atheroma preparations; Anti-Epileptics; Psychoanaleptics Excluding Anti-Obesity Preparations; Psycholeptics
-
Originator :
Johnson & Johnson
-
CAS No. :
97240-79-4
-
Trade Name. :
TOPAMAX
-
Molecular Weight :
339.363 g/mol
-
Molecular Formula :
C12H21NO8S
Application
Topiramate is used alone or with other medications to prevent and control seizures (epilepsy). This medication is also used to prevent migraine headaches and decrease how often you get them. Topiramate will not treat a migraine headache once it occurs.
General Description
Topiramate (brand name Topamax) is a broad-spectrum anticonvulsant (antiepilepsy) drug. In late 2012, topiramate was approved by the United States Food and Drug Administration (FDA) in combination with phentermine for weight loss. The drug had previously been used off-label for this purpose. Topiramate was originally produced by Ortho-McNeil Neurologics and Noramco, Inc., both divisions of the Johnson & Johnson Corporation. This medication was discovered in 1979 by Bruce E. Maryanoff and Joseph F. Gardocki during their research work at McNeil Pharmaceuticals.[1][2][3] The commercial usage of Topiramate began in 1996.[4] Mylan Pharmaceuticals was granted final approval by the FDA for the sale of generic topiramate in the United States and the generic version was made available in September 2006.[5] The last patent for topiramate in the U.S. was for use in children and expired on February 28, 2009.