- February-2-2022
Finished formulation manufacturers: Guidelines for potent drug process
Finished formulation manufacturers and suppliers in Mumbai, the value chain of a pharma industry is primarily related to the manufacture of APIS and finished formulations. Both are essentially different from the other. The finished formulations distinctly deal with the potent drug process. It is a blend of diverse chemicals and the active ingredient is measured in ratio to make a drug. API is the drug dosage or the key element that makes the drug fit for consumption. Globally, large pharma companies have a network of manufacturers who work on finished formulations.
Oceanic Pharmachem based in India supplies finished formulations for several pharma companies across continents. Their business trail consists of a blend of experienced and specialized professionals who follow stringent guidelines for the potent drug process. This post focuses on the processes that make drugs available in the international market.
Formulations impression
Drug manufacture is becoming a complex process primarily because some diseases call for trials. For example, oncology is one area where combinations of drugs are used for patients. They are now being targeted with the intention of interaction with biological and pharmacological activities. The potency of high drugs calls for minute formulations of substances. Manufacturers need to pay more attention to ratios and values. They also need to protect those who handle materials so the drugs are safe for patient usage in various parts of the world. To make an entire batch, very small quantities of drug per unit dose is used during the production.
Why is safety critical?
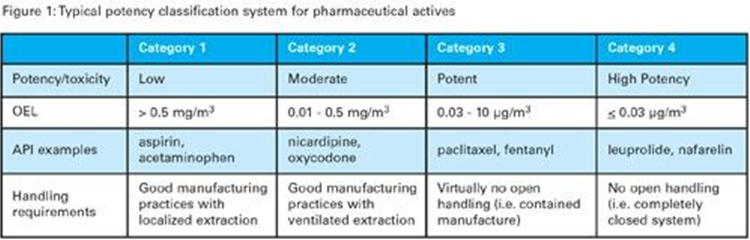
In the manufacturing plant many toxic substances are used and workers need to handle them with care. PPE is used to avoid contamination at source for materials and human resources. Advanced isolator technology is applied to stop exposure from toxic compounds. It also enables manufacturers to decide the ratios of the drug’s raw materials for blending. Most drug substances are classified according to their potency levels. The occupational exposure limits determine safety standards in the plants.
Determining factor for high potency
If the compound dose is less than 1 mg then it is considered ‘highly potent’. One example of this is digoxin (Linoxin capsules 100ug per capsule). Manufacturers have to take up the challenge of producing the formulation in bulk. The batches are then ready for dispatch to offshore customers. The drug content per unit is in very tiny quantities. Swab samples are taken for cleaning the equipment for verification purposes. Then the formulation is produced as a liquid so that it dissolves in the right capsule or gel.
Semi-finished formulations techniques
Under different processes intermediates are produced by blending various pharma ingredients. The correction ratios are critical in making the final product. In the case of a semi-finished formulation one active ingredient is present for the desired effect to treat patients. When processed it is pure and remains unadulterated. It is an easier process to handle and can produce products in various forms like medicated powder, liquid, spraysand capsules.
Oceanic Pharmachem is an end-to-end manufacturing company for finished formulations. We follow all guidelines by approved international medical authorities like FDA to make highly potent drugs. Talk to us for your targeted patient needs.

.PNG)